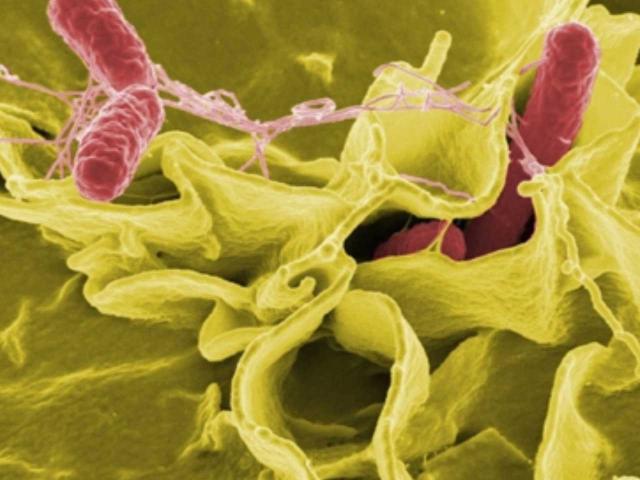
Research
Pathogenesis / Immunity
Salmonella is the greatest foodborne-disease burden in the United States— causing a million illnesses annually. Our research is focused on molecular mechanisms underlying Salmonella pathogenesis. We have shown that Salmonella virulence gene expression is governed by the DNA adenine methylase (Dam). Salmonella dam mutants are attenuated for virulence, modify cytokine responses in infected hosts, and promote protective immunity in vaccinated animals. Salmonella dam vaccines are currently undergoing clinical trials in livestock to improve animal health and food safety. Our collaborators include D. Low (UCSB), C. Samuel (UCSB), R. Daynes (Utah), and J. House (Sydney).
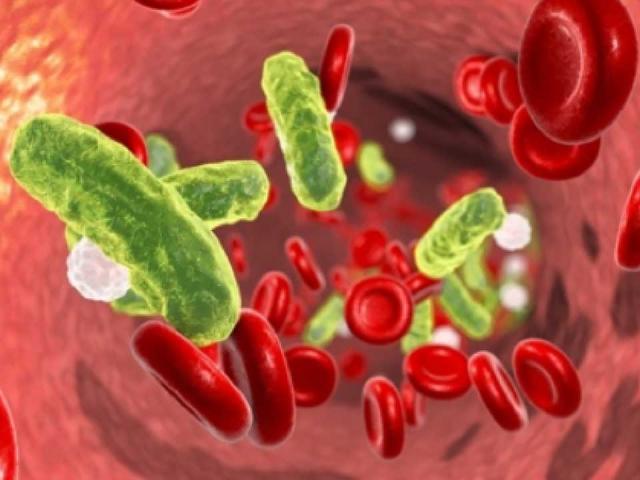
Bacterial Sepsis
Sepsis is a life-threatening bloodstream infection resulting in inflammation, coagulation and organ failure. Although it is the leading cause of death in non-cardiac intensive care units, no new effective therapies have been developed in decades. Comparative studies of the onset and progression of sepsis caused by distinct Gram-positive and Gram- negative bacterial pathogens revealed a unique mechanism of host protection against lipopolysaccharide (LPS) toxicity of Gram-negative pathogens. Toll-like receptor 4 (TLR4)-dependent processes result in increased neuraminidase (NA) activity that increased the clearance of circulating anti-inflammatory alkaline phosphatase (AP), diminishing LPS de-phosphorylation and increased LPS toxicity via increased TLR4-dependent inflammation. Therapeutic reversal of Gram-negative sepsis was achieved by administration of NA inhibitors or AP. Our collaborators include J. Marth (UCSB), V. Nizet (UCSD), and J. Smith (SBP, La Jolla).

People Are Not Petri Plates: Why Antibiotics Fail
There is an urgent need to identify antibiotics capable of killing multidrug-resistant (MDR) pathogens. However, the identification of antimicrobials capable of killing MDR pathogens is hindered by a fundamental flaw in the paradigm for antimicrobial susceptibility testing (AST): it does not take into account host-pathogen interactions that can have profound impacts on microbial susceptibility. Antibiotics that pass AST often fail in patients, while other efficacious antibiotics are excluded because they failed standard testing. By developing AST methods that consider microbial-host interactions, we have identified antibiotics that clear MDR pathogens from infected animals. These novel methods enable more accurate antibiotic prescription and are expected to improve patient outcomes. Our collaborators include J. Smith (SBP, La Jolla), J. Marth (UCSB), and J. House (Sydney).

Rapid and Affordable UTI Diagnosis
Urinary tract infections (UTIs) affect nearly 10 million people in the U.S., annually— mostly women. Urine screening for bacteria in pregnancy remains one of the most effective, and cost-effective interventions in the world of infectious diseases. Unfortunately, in many underserved clinics, routine culture is not readily available due to inadequate resources, highlighting the need for point-of-care testing with real-time treatment. We have developed a rapid and affordable smartphone-based system for UTI diagnosis and plan to use this system as a point-of-care device for UTI screening at local clinics. Our collaborators include T. Soh (Stanford), J. Marth (UCSB), L. Fitzgibbons, A. Zambrano, and J. Fried (Cottage Hospital).

Hypervirulent Bacteria
‘Hypervirulent’ Salmonella isolated from natural microbial populations are among the most virulent microbes encountered of this species. These strains are 100-times more virulent and are more capable of killing vaccinated animals. Hypervirulent Salmonella utilize a ‘Trojan Horse’ strategy whereby virulence functions are only revealed during the infective process. Resultant actin cytotoxin and inflammatory cytokines confer profound effects on microbial virulence and the capacity of the host to mount an effective immune response. Hypervirulent strains of other pathogens are likely present among natural microbial populations, posing a previously unrecognized risk to human health. Our collaborators include Bart Weimer (UCD) and J. House (Sydney).
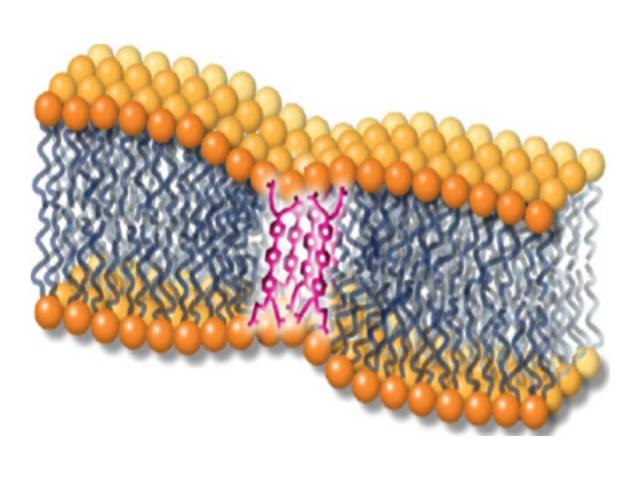
Novel Antimicrobials
Conjugated oligoelectrolytes (COEs) are a class of small hydrophobic molecules that were initially designed to integrate into the bacterial membrane and facilitate electron transfer for the development of microbial fuel cells. COEs have low toxicity in mammalian cells, are well-tolerated in mice, and confer protection against virtually untreatable infections in mice (e.g., MRSA and carbapenem-resistant K. pneumoniae). COE’s provide a potential means to treat multidrug-resistant (MDR) infections for which current therapeutic options are limited and lacking in the pipeline. We plan to develop these compounds into a new antimicrobial class, with a particular emphasis on efficacy against MDR pathogens. Our collaborators include G. Bazan (UCSB), J. Hinks (Singapore), and D. Low (UCSB).
Future Plans
We plan to continue our investigation into the molecular mechanisms underlying microbial pathogenesis and the associated innate and adaptive immune responses that increase disease and compromise host immunity. The applied goal is to translate this research into effective therapeutic strategies for the treatment of multidrug-resistant pathogens and microbial sepsis (e.g., antimicrobials, sepsis therapeutics, vaccines).